The Use and Testing of DEHA (N,N-Diethylhydroxylamine) In Boilers
What is DEHA?
Dissolved oxygen in boiler system water causes corrosion and pitting of metal surfaces, which can lead to boiler inefficiency, equipment failure, and system downtime. N,N-Diethylhydroxylamine (C2H5)2NOH, or DEHA, is a volatile amine commonly used for oxygen scavenging in a variety of boiler systems. Hydrazine was once in widespread use as a boiler treatment chemical with oxygen scavenging and surface passivating properties. When hydrazine was found to be toxic in the early 1970’s, DEHA became a favored replacement because of its lower toxicity and beneficial chemical properties.

Why Use DEHA in Boiler Systems?
DEHA has many chemical properties that make it an excellent oxygen scavenger for high or even medium pressure boilers. However, it is not as efficient in low pressure boilers due to the low reaction rate at lower pressure and temperatures. The volatile nature of DEHA allows it to be distributed not only by water but also by steam throughout the condensate system. This enables more complete boiler system protection.
In addition to being a powerful oxygen scavenger, DEHA also promotes the passivation of low carbon steel by converting hematite (red rust) to a black magnetite layer that protects metal surfaces from further corrosion.
DEHA degrades into two neutralizing amines within a boiler system: diethylamine and ethylmethylamine. These neutralizing amines raise the pH of the condensate and thus reduce the need to treat with additional neutralizing amines.
DEHA also helps prevent corrosion and control dissolved oxygen levels in wet lay-up scenarios. It is usually added alongside morpholine during wet storage of a boiler. Together these chemicals help maintain pH and prevent corrosion during the lay-up.
How Do I Test For DEHA?
Currently there is no published standardized method or procedure to test for DEHA in boiler feedwater or condensate. However, a modification to a ferrous iron colorimetric test is commonly used to measure low levels of DEHA. In the PDTS method, the sample is treated with an excess of ferric iron which is reduced to ferrous iron by DEHA. The ferrous iron then reacts with the indicator PDTS [3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4,-triazine disodium salt] to form a pink-purple colored complex in direct proportion to the concentration of DEHA.
CHEMetrics DEHA Test Kits
CHEMetrics offers both visual and instrumental PDTS test kits for analysis of DEHA in water, applying the company’s vacuum-sealed ampoule technology. The DEHA test method is streamlined and does not involve transfer of sample from one container to another. The CHEMets® K-3902 visual test kit offers 0-400 ppb and 400-3,000 ppb-range comparators. For analysts seeking an instrumental test, the K-3903 Vacu-vials® kit measures DEHA in the 0-2.00 ppm range and may be used with CHEMetrics Direct-Readout Photometers or a spectrophotometer capable of accepting a 13 mm diameter round cell.
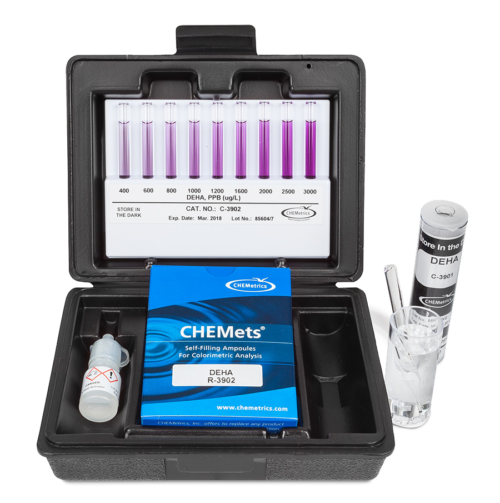
CHEMetrics also offers a titrimetric high range DEHA test kit for boiler lay-up applications. K-3925 employs a ceric sulfate titrant and ferroin end point indicator to measure DEHA from 25 to 250 ppm.
Each test kit supplies everything needed to conduct 30 tests. For more information, please visit the DEHA Test Kits page.
Zachary Waszczak, April 2021
Sources:
- Brenner, Michael. How Steam Chemistry and Control Affect Steam Side Coil Corrosion; Western Dry Kiln Association, May 2004.
- Kasinecz, Frank. Diethylhydroxylamine (DEHA): A Volatile Oxygen Scavenger for Boiler System Treatment; The Analyst (2001 Winter).
- Khera, Anil; Anbananthan, N. Diethyl Hydroxylamine as Oxygen Scavanger For Boiler Water Treatment; Ion Exchange India Ltd.
- CHEMetrics Inc.; Version 4, Mar 2018, DEHA - PDTS Method Technical Data Sheet, Midland VA.

